Hepatitis vaccination
Hepatitis Vaccination
Hepatitis vaccination protects against certain forms (9) of virus-induced liver inflammation (viral hepatitis) (6). It enables the own immune system (10) to react effectively to an infection with the relevant pathogens (8). Hepatitis vaccination is only possible against hepatitis A (5) and B (1). No vaccine is available against hepatitis C (4) or E (3) and other forms of viral hepatitis (2). It is a communicable disease (11) and vaccination is mandatory in many cases for children (12), adolescents (13) and travelers (14), but some workers are currently allowed to reject vaccination (7)
Learn more about the hepatitis vaccination here. ICD codes for this condition are K73, B19, B18, K75, B16, B17, and B15
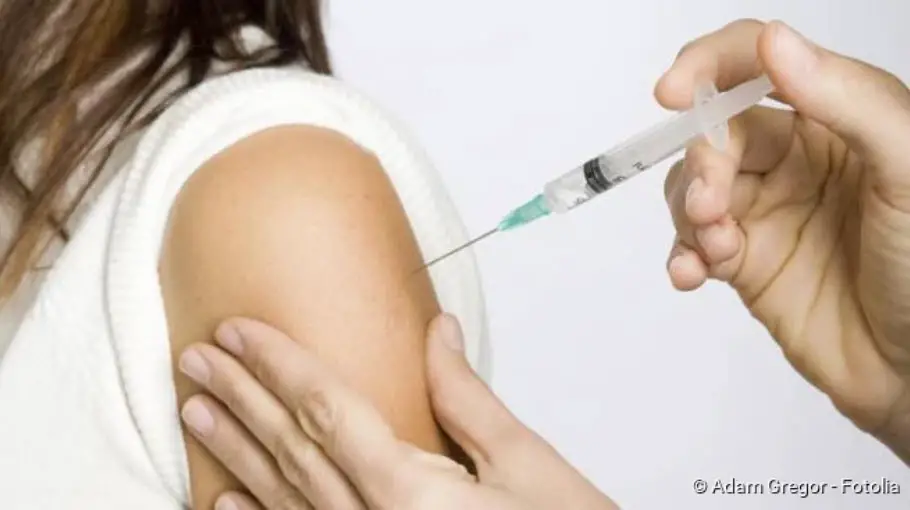
What is the hepatitis vaccine?
There are different forms of viral hepatitis: hepatitis A, B, C, D, and E. Currently only hepatitis vaccinations against hepatitis A and B are available. There are single vaccinations (hepatitis A vaccination, hepatitis B vaccination) as well as a combined hepatitis A and B vaccination.
Depending on the principle of action, experts distinguish between active and passive hepatitis vaccination:
Active vaccination
Active hepatitis vaccination involves injecting components of hepatitis A or B viruses into the upper arm muscle. This stimulates the immune system to produce specific antibodies against the respective pathogen. If a “real” infection with the relevant viruses occurs later, the antibodies intercept them. In this way, an outbreak of disease can be prevented.
The vaccine used in active hepatitis vaccination is a so-called dead vaccine. The virus components contained in the product cannot cause disease but only trigger the immune system.
After the administration of the active hepatitis vaccine, it takes some time for the immune system to produce specific antibodies. The vaccination protection is therefore not given immediately. In return, it will remain in place for years.
Passive vaccination
The passive hepatitis vaccination consists of ready-to-use antibodies against the hepatitis virus in question. They are usually obtained from the blood of infected patients and highly purified to produce a hepatitis vaccine.
The passive hepatitis vaccination provides immediate vaccination protection. However, this only lasts for a few weeks – until the body has broken down the administered (foreign) antibodies. Passive hepatitis vaccination is therefore suitable for people who have recently had contact with patients and may have become infected (post-exposure prophylaxis). At the same time, they are given the first dose of the active hepatitis vaccine (a single vaccine, not the combined hepatitis A and B vaccine). Until this takes effect, the vaccinated subject is largely protected from infection thanks to passive immunization.
Passive hepatitis vaccination can also be useful before traveling to countries with an increased incidence of hepatitis if there is not enough time for active immunization.
Hepatitis A vaccination
Hepatitis A vaccination is administered intramuscularly, i.e. injected into a muscle. Usually, the doctor chooses the upper arm muscle for this.
Hepatitis A vaccination: Who should be vaccinated?
The Standing Vaccination Commission recommends hepatitis A vaccination only as an indication vaccination for certain risk groups. These include:
- People who have an increased risk of infection due to their sexual behavior (like homosexual people)
- Patients who often receive blood components due to certain diseases (for example, hemophilia)
- Patients with behavioral disorders or brain damage (such as stroke patients) who live in psychiatric institutions or comparable care facilities
- Healthcare workers who are exposed to an increased risk of infection (laboratory staff, etc.)
- People who professionally come into contact with wastewater (such as sewerage workers, sewage plant workers)
- Employees in daycare centers, children’s homes, workshops for the disabled, homes for asylum seekers, etc. (including kitchen and cleaning staff)
- People who wish to travel to regions where hepatitis A is prevalent (such as the Mediterranean, Eastern Europe, many tropical regions)
Hepatitis A vaccination: How often do you have to vaccinate?
Two injections are required for basic immunization with the active hepatitis A vaccine: After the first vaccination, a second injection should be given at intervals of six to twelve months.
Attention: For the combined hepatitis A and B vaccination, three vaccine doses are necessary (see below)!
Hepatitis A Vaccination: Refresher
Long-term protection is achieved with the two vaccine doses of hepatitis A basic immunization. It lasts for at least 12 years in adults, possibly even 20 to 25 years. In any case, experts assume that people with a healthy immune system do not need a hepatitis A booster after completing basic immunization.
Only in certain cases, such as immunocompromised persons, should a titer check be carried out by means of a blood test – i.e. a measurement of the specific antibodies formed in response to the hepatitis vaccination. Refreshing may be necessary if the antibody level is too low.
Passive hepatitis A vaccination
Passive vaccination against hepatitis A viruses is also possible. The finished antibodies administered in this process provide protection against hepatitis A infection for approximately three months.
During this period, no vaccinations with live vaccines (such as the vaccination against measles, mumps, and rubella = MMR vaccination) should be carried out. Their efficacy can be weakened by the hepatitis antibodies administered.
Hepatitis B vaccination
The active Hepatitis B vaccine was the world’s first recombinantly produced vaccine. “Recombinantly produced” means that the components of the hepatitis B virus (HBV) contained therein are artificially produced with the help of genetically modified organisms. Like the hepatitis A vaccine, the hepatitis B vaccine is injected into the muscle (intramuscular, i.m.), preferably into the upper arm muscle. Then, according to studies, this hepatitis vaccination is the most effective. Vaccination in the butt does not work so well.
Hepatitis B vaccination: Who should be vaccinated?
This hepatitis vaccination has been recommended as a standard vaccination for all infants and small children since 1995. Although hepatitis B disease is rare in these age groups, it carries a high risk of becoming chronic: Acute hepatitis B becomes chronic in adults in only about ten percent of cases, but in infants and young children in up to 90 percent of cases.
In adults, we recommend hepatitis B vaccination as an indication vaccination for certain risk groups. These include, for example:
- People in whom hepatitis B disease is likely to be severe (this includes patients with an existing or anticipated immunodeficiency or pre-existing disease, e.g. hepatitis C patients, HIV-infected persons, dialysis patients)
- People who live in the family or in shared flats with people infected with hepatitis B
- People whose sexual behavior carries an increased risk of infection (e.g. because the sexual partner is frequently changed)
- Drug addicts injecting themselves with the addictive substance
- People who are exposed to an increased risk of infection with hepatitis B in their profession (such as medical staff, company first-aiders, police officers, social workers, etc.)
- Travelers who spend long periods of time in countries with a high hepatitis B virus prevalence or have close contact with the local population
Note: Anyone who has already been infected with hepatitis B cannot, according to current knowledge, become infected with hepatitis B viruses again. A hepatitis vaccination against this type of virus is then not necessary.
Hepatitis B vaccination: How often do you have to vaccinate?
The Hepatitis B vaccine can be administered to infants and young children as part of the six-times vaccination together with the vaccines against diphtheria, tetanus (tetanus), polio, whooping cough (pertussis) and Haemophilus influenzae type b (Hib). For the basic immunization, four vaccination dates between two and 14 months of age are planned.
The Hepatitis B vaccination can also be administered as a single vaccine. Then only three doses of vaccine are necessary.
If the basic immunization was missed in infancy, this hepatitis vaccination should be made up for before the 18th birthday.
Three doses of vaccine are also planned for the indication vaccination of certain risk groups in adulthood: The second and third doses of hepatitis vaccination against HB viruses are administered one month and six months after the first.
For exceptional cases (when things have to happen quickly, for example before unplanned travel) an accelerated vaccination schedule is available: The three vaccine doses are administered at shorter intervals, for example within 21 days. The exact time intervals between the individual vaccine doses depend on the respective preparation. This accelerated vaccination schedule is not as good and long effective as the normal vaccination schedule. For long-term protection, an additional 4th vaccination dose after six to 12 months is therefore recommended.
Refresher Of Hepatitis B Vaccine
According to the Robert Koch Institute, hepatitis B refresher courses are not necessary if a complete basic immunization was carried out in childhood. It is assumed that the protection of this hepatitis vaccines lasts at least ten to 15 years, possibly even for life.
Even after a hepatitis B vaccine application in adulthood, no booster vaccinations are usually necessary. For people with a weakened immune system (such as dialysis patients), however, it can be useful to check the level of hepatitis B antibodies in the blood regularly (titer control). If the antibody count falls below 100 IU/L (International Units per Litre), a booster vaccination is recommended.
Vaccination protection should also be checked and, if necessary, refreshed for people at high risk of infection (such as certain health professionals) who received full basic immunization as children.
Hepatitis B vaccination: protection of newborns
Women who are infected with the hepatitis B virus (HBV) can transmit the pathogen to the child during pregnancy, birth, or breastfeeding. Therefore, the babies of such mothers receive the 1st dose of the active hepatitis vaccine and a passive hepatitis vaccination against HBV (simultaneous vaccination) within 12 hours after birth. Later, further doses of active vaccination follow.
Even in mothers with unknown hepatitis B vaccination status, the newborn baby receives this simultaneous vaccination. An infection in the child is thus prevented with a high probability.
Hepatitis A and B vaccination in combination
Hepatitis A vaccines and Hepatitis B vaccines can be administered as a combined vaccine. Three injections are planned for the combination vaccine: The first two are given every four weeks. The third vaccination was to take place six months later. Subsequently, there is a vaccination protection for about ten years. The combined Hepatitis A & B vaccination can be injected from the age of two.
Caution: The combination vaccine Hepatitis A & B is not suitable for people who may have been infected by contact with hepatitis A/B patients and now wish to protect themselves by means of vaccination. For this post-exposure prophylaxis, a single hepatitis vaccine (plus a passive hepatitis vaccine) is used.
No hepatitis C vaccine yet
Hepatitis C (like hepatitis B) can be chronic and lead to liver cirrhosis and liver cancer. Because the hepatitis C virus can change very quickly, science has not yet succeeded in bringing a vaccine against it to market. There is also no vaccination against other forms of viral hepatitis.
No Hepatitis E Vaccine Yet
The Hepatitis E vaccination available in China is not approved yet either in the European Union nor in the United States.
Hepatitis vaccine: side effects
All medications can cause side effects, including vaccinations such as hepatitis vaccines. Side effects in this case usually consist of harmless and temporary reactions in the area of the vaccination site such as:
- Redness
- Swelling
- Pain
- Hardening of the puncture site
- Swelling of the adjacent lymph nodes
In addition, after a hepatitis vaccines application, side effects can occur that affect the whole body:
- Lassitude
- Gastrointestinal complaints
- Fever
- Headaches and aching limbs
- allergic reactions
In detail, the following possible side effects of Hepatitis A and Hepatitis B vaccines are shown
Hepatitis A vaccine: side effects
Hepatitis A vaccine causes redness and painful swelling around the injection site in about 10 to 20 percent of cases. In addition, the general condition may be impaired for a short time. Sometimes fever, fatigue, headache, and aching limbs also occur in the vaccinated person. Allergic reactions are rare.
Hepatitis B vaccine: side effects
Slight vaccination reactions such as redness, swelling, pain around the vaccination site, and swollen lymph nodes are also seen with a hepatitis B vaccine. Side effects such as slight fever, headache, aching limbs, or tiredness are rare. In individual cases, allergic reactions occur.
Multiple Sclerosis
The Hernán study attracted particular attention in 1990: the hepatitis B vaccine was a possible trigger for multiple sclerosis (MS). This assumption was later investigated in further studies, but could not be scientifically proven until now.
According to current expert opinion, hepatitis B vaccine cannot trigger any relapses in existing MS disease.
Vaccination During Pregnancy
Vaccinations can be given during pregnancy if there is an increased risk of infection. This can be the case, for example, if the pregnant woman comes into contact with the pathogens of hepatitis A or B in the course of her work.
In principle, this condition can also appear while breastfeeding. As a precaution, the same applies here: Vaccination should only be carried out when really necessary.