Coronavirus – Covid 19 Complete Reference
COVID-19 [3], [4] also known as coronavirus disease [3] or, incorrectly, coronavirus pneumonia, is an infectious disease caused by the SARS-CoV-2 virus.9][10] It was first detected in the Chinese city of Wuhan (Hubei Province) in November 2019. [11][12] Within three months it spread to virtually all countries in the world, leading to the World Health Organization’s declaration of a pandemic.
It produces flu-like symptoms, including fever, cough, shortness of breath, myalgia and fatigue. 14][15] Sudden loss of smell and taste (not caused by mucus) has also been observed. 16] In severe cases, it is characterized by pneumonia, acute respiratory distress syndrome,[17] sepsis,[18] and septic shock that leads to about 3% of those infected dying. There is no specific treatment; the main therapeutic measures are to relieve symptoms and maintain vital functions. [14]
The most frequent form of human-to-human transmission, airborne, is due to the small droplets (known as Flügge microdrops [19]) that are emitted when talking, sneezing, coughing or breathing out.
The routes of person-to-person transmission of SARS-CoV-2 include direct transmission, such as coughing, sneezing, droplet transmission, and contact transmission, such as contact with oral, nasal, and ocular mucous membranes. [20]
Symptoms appear between (2) two and (14) fourteen days, with an average of (5) five days after exposure to the virus. 21][22][23][24] Transmission can be prevented by hand washing, use of masks, coughing up bleeding (sunken part of the arm opposite the elbow), and early diagnosis of the disease. [25][15]
The World Health Organization announced on 11 February 2020 that COVID-19 would be the official name of the disease. The name is an acronym for coronavirus disease 2019. Care was taken to ensure that the name did not contain references to any place, animal species or group of people in line with international recommendations, to avoid any stigmatization against any group. [26]
Covid-19 was first identified on December 1, 2019, in the city of Wuhan, capital of Hubei Province, in central China, when a group of people were reported to have pneumonia of unknown cause, linked mainly to workers at Wuhan’s southern Chinese wholesale seafood market. 27] The number of cases increased rapidly in the rest of Hubei and spread to other territories.
The rapid spread of the disease led the World Health Organization to declare it a health emergency of international concern on 30 January 2020, based on the impact the virus could have on underdeveloped countries with less health infrastructure, [28] and to recognize it as a pandemic on 11 March. 13] Cases have been declared in Western countries (including Italy, Spain and the United States, in terms of the number of patients[29]), in the Asia-Pacific region and in almost all parts of the world. [30]
To prevent the spread of the virus, governments have imposed travel restrictions, quarantines, confinements, social isolation, cancellation of events, and closure of establishments. The pandemic is having a disruptive socio-economic effect,[33] and fears of supply shortages have led to panic buying. There has been misinformation and conspiracy theories spread online about the virus,[34][35] and incidents of xenophobia and racism against citizens of China and other East and Southeast Asian countries. [36]
The cause of covid-19 is infection with the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [9][37] which is a type of Orthocoronavirinae. 3][9] Initially, the virus was named 2019-nCoV[38]. It was first discovered and isolated in Wuhan, China, after causing the 2019-2020 coronavirus disease epidemic. It appears to be of zoonotic origin, i.e., it passed from an animal host (a bat) to a human host. [39]
The virus genome consists of a single strand of RNA, so it is classified as a single-stranded RNA-positive virus. Its genetic sequence has been isolated from a sample obtained from a patient affected by pneumonia in the Chinese city of Wuhan. [40][41][42][43][44][45]
It was suggested that interpersonal transmission of the virus could occur through saliva droplets expelled through coughing and sneezing. 14] It can cause acute respiratory disease and severe pneumonia in humans.
It all starts inside the human body, when the coronavirus comes into contact with the mucous membranes of the nose, eyes or mouth from respiratory secretions of an infected person or from contact with hands contaminated by the virus. It is there, mainly in the cells of the mucosa of the upper respiratory tract, that the virus has the opportunity to enter as if it were a Trojan horse. The microscopic infiltration occurs because this virus has developed specific “keys” (proteins) that serve to open the “locks” of these human cells and have a free way to infiltrate them. One of the keys it uses, the S-protein, fits very well with a lock, the ACE2 protein, which is present on the surface of a wide variety of cells in the human body. [46]
Once the coronavirus has managed to enter the cells, it takes over the cellular machinery to use it for its own benefit. It forces the human body’s own cells to make millions and millions of copies of the virus by synthesizing its RNA (its genetic material) and its proteins which are then assembled to create new viruses. These new viruses leave the cells, destroying them, and go to neighbouring cells to repeat the cycle indefinitely.
The SARS-CoV-2 virus is able to spread and multiply in the cells of the respiratory tract (also in other types of cells to a lesser extent, such as intestinal cells), without our body sending out alarm signals for several days. This is what we call the incubation period, the time that passes from the moment of infection until the development of symptoms; the calm before the storm.
The WHO estimates that the incubation period (the time between infection and the onset of symptoms), lasts between two and ten days,[47] while for the US Centers for Disease Control and Prevention it lasts between two and fourteen,[48] although it is generally 4-6 days. 49][50] A study published in February by Chinese researchers found that the period can be extended to 24 days. 51] One case is reported to have had an incubation period of 27 days. [52]
Infected people may be asymptomatic or have a flurry of symptoms ranging from mild to very severe, including fever, cough and shortness of breath. 53][54][55] Diarrhea and other rhinopharyngeal symptoms, such as sneezing, runny nose, and sore throat, are less common. [56]
The symptoms of covid-19 are non-specific and its presentation, according to WHO/WHO, may even be symptomless (asymptomatic). Based on a statistical sample of 55 924 laboratory-confirmed cases, the frequency of presentation of the symptoms in the Chinese population was as shown in the attached table. [57]
| Symptom present: | Frequency *
(%) |
|---|---|
| Fever | 87,9 % |
| Dry cough | 67,7 % |
| Fatigue | 38,1 % |
| Sputum production | 33,4 % |
| Dyspnea | 18,6 % |
| Muscle pain or joint pain | 14,8 % |
| Sore Throat | 13,9 % |
| Headache | 13,6 % |
| Chills | 11,4 % |
| Nausea or vomiting | 0 5,0 % |
| Nasal congestion | 0 4,8 % |
| Diarrhoea | 0 3,7 % |
| Hemoptysis | 0 0,9 % |
| Conjunctival congestion | 0 0,8 % |
*(Until 1 May 2020 and based on 55,924 confirmed cases per laboratory). [57]
Symptoms were reported to include fever in 90% of cases, fatigue and dry cough in 80% of cases, and breathing difficulties in 20% of cases. 58][59][60][61] Chest x-rays have revealed signs of pneumonia in both lungs. Vital signs are generally stable during the time of hospitalization. Blood tests have shown a low number of white blood cells in the blood.
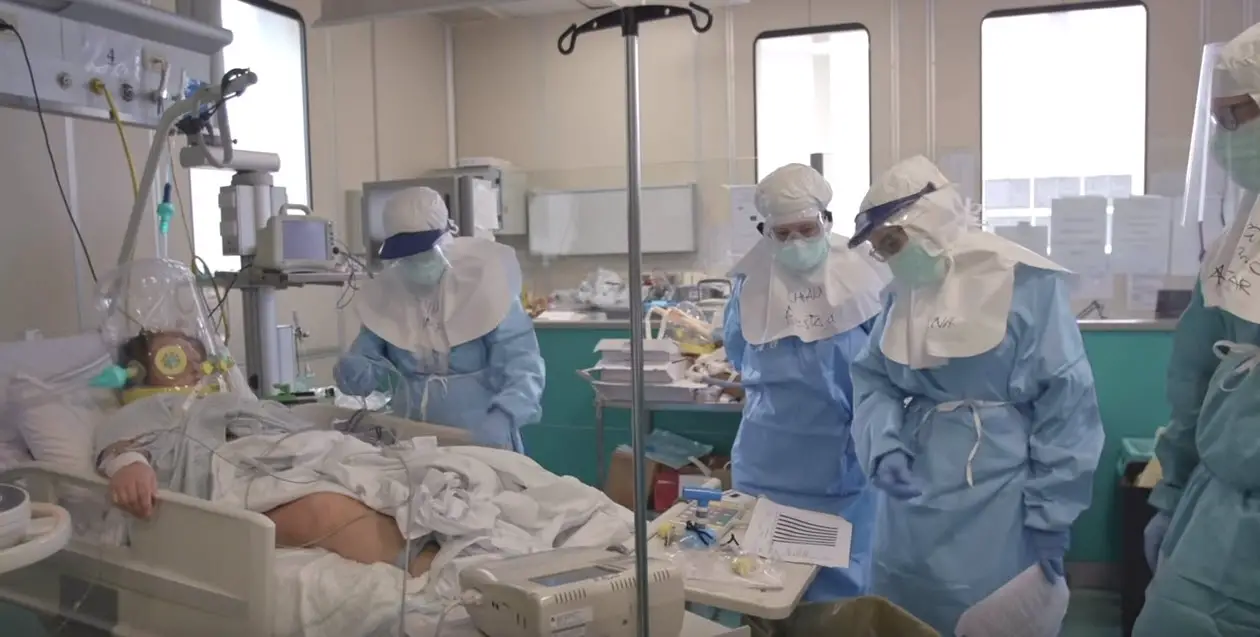
On 20th January The Lancet published a study of the first 41 cases of patients admitted with a confirmed diagnosis from 16th December 2019 to 2nd January 2020. 62] Of these, less than half had underlying diseases, including diabetes, hypertension, and cardiovascular disease. Common symptoms at the onset of the disease were fever, cough, and myalgia or fatigue; less common symptoms were sputum production, headache, hemoptysis, and diarrhea. Dyspnea developed in 22 out of 40 patients (55 %), with a median time from disease onset to dyspnea of 8 days. Lymphopenia was present in 26 out of 41 patients (63 %). All patients had pneumonia with abnormal findings on chest CT. Complications included acute respiratory distress syndrome (positive for real-time RT-PCR in the plasma sample), acute cardiac injury and secondary infection. Thirteen patients (32%) were admitted to an ICU and six died (15%). In a clinical commentary in the same journal, a comparison of clinical presentation against other emerging coronaviruses (SARS and MERS) is presented; among other clinical data in the cases studied so far, it should be noted that upper respiratory tract symptoms are remarkably uncommon (e.g. no patient had a sore throat). [63]
Some of those infected may be asymptomatic and show test results that confirm the infection but do not show clinical symptoms, so researchers have issued advice that people in close contact with confirmed infected patients should be monitored and examined closely to rule out infection. [64]
Clinical Phase
The spread of the coronavirus throughout the body alerts the immune system, which responds through multiple and complex mechanisms to stop its proliferation and attack the infected cells. It is the beginning of the clinical phase of the disease, where the symptoms and signs that a person suffers are caused not only by the action of the coronavirus, but also by the human body’s defense systems against it, through mechanisms such as inflammation. The symptoms usually begin in the upper airways and progressively move down through the bronchi until, in some people, they reach the lungs.
The disease that causes the new coronavirus, COVID-19, can present clinically with very varied signs and symptoms depending on the characteristics of the person. However, dry cough (68% of affected patients), fever (88%) and respiratory distress (19%) are three key signs of suspected new disease. Other very common symptoms, as recorded by the World Health Organization (WHO), are general fatigue (38%), expectoration (33%), sore throat (14%), headache (14%), muscle or joint pain (15%), chills (11%), nausea or vomiting (5%), nasal congestion (5%), diarrhea (4%) or coughing up blood. In addition, many health professionals have also observed that some affected people lose their sense of smell and taste for several days. It is during the clinical phase that the maximum release of virus from the respiratory mucosa occurs, although this may also occur, to a lesser extent, in an asymptomatic stage or in the recovery process.
In reality, symptoms such as fever or headache are not caused by the virus, but by the immune response of the human body to fight the virus. Something similar occurs with death, it is not only the coronavirus that causes death (parasites are not usually interested in killing their hosts), but in some cases it is also caused by an uncontrolled immune response (called “cytokine storm”) that can lead to multiorgan failure. Although it is not yet known how, the coronavirus can trigger a disproportionate inflammation that causes more harm than good to the patient.
The most common complications are pneumonia and multiorgan failure, which sometimes result in death. [11][65]
When the virus manages to travel to the lungs and the immune system responds, viral pneumonia occurs, the main nightmare for health professionals in this pandemic. This pneumonia can cause anything from mild respiratory distress to outright suffocation due to the inability of the lungs to deliver oxygen to the blood. The virus interferes with this process by damaging the cells of the lungs and causing severe inflammation that clogs these organs, interfering with their gas exchange function.
Fortunately, in 80% of the cases of COVID-19 the disease is mild, to the point of being confused with colds or flu. However, 15% of patients show severe symptoms that require hospitalization and 5% develop very serious symptoms that must be treated in intensive care units.
Clinical research found that a high concentration of cytokines is detected in the plasma of critically ill patients infected with SARS-CoV-2, suggesting that the cytokine storm was associated with the severity of the disease. [66]

Laboratory equipment needed to perform real-time PCR, a technique for the diagnosis of infection.
Guidelines published on February 6 by the Zhongnan Hospital of Wuhan University recommended methods based on epidemiological risk and clinical characteristics for diagnosis. This included identifying patients who had recently traveled to Wuhan or had had contact with someone infected, as well as two or more of the following symptoms: fever, radiological signs of pneumonia, normal or low white blood cell count (leukopenia) and lymphopenia. [67]
WHO published several protocols for the diagnosis of the disease for Japan. 68][69] The test of choice was real-time RT-PCR (or retrotranscription followed by quantitative polymerase chain reaction). 70] It was performed on respiratory or blood samples. 71] Results were available, as of January 30, within a few hours or days. 72][73] The PCR test can be performed because Chinese scientists have isolated and published a genetic sequence of the coronavirus. [11][74][75][76][77]
Chinese scientists succeeded in isolating a strain of the coronavirus and publishing the genetic sequence so that laboratories around the world could independently develop PCR tests to detect infection by the virus. [78][79][80][81]
Preventing an acute peak of infections, known as flattening the epidemic curve, helps prevent health systems from collapsing, and also provides more time for treatment/vaccine development. Spreading infections over a longer time frame allows health services to better manage patient volumes. [82][83]
There is no known effective treatment for the disease. The WHO recommends that randomised controlled trials be conducted with volunteers to test the effectiveness and safety of some potential treatments. [84]
Research to find an effective treatment began in January 2020, but results are not likely to be available until 2021. [85] The Chinese Center for Disease Control and Prevention began testing the effectiveness of some pre-existing effective treatments for pneumonia in patients with COVID-19 in late January. [90]
Investigational treatments
Vaccines
Three vaccination strategies are being investigated. First, researchers aim to build a whole virus vaccine. The use of such a virus, either inactive or killed, aims at a rapid immune response of the human body to a new infection with COVID-19. A second strategy, subunit vaccines, aims to create a vaccine that sensitizes the immune system to certain subunits of the virus. In the case of SARS-CoV-2, this research focuses on the S-spike protein that helps the virus introduce the enzyme ACE2. A third strategy is nucleic acid vaccines (DNA or RNA vaccines, a novel technique for creating a vaccine). Experimental vaccines of any of these strategies would have to be tested for safety and efficacy. [91]
Several organizations in different countries are in the process of developing a vaccine. The U.S. National Institutes of Health hopes to conduct human trials of a vaccine by April 2020. [92] The Chinese Center for Disease Control and Prevention (CCDC) has begun developing vaccines against the new coronavirus and is testing the effectiveness of existing drugs for pneumonia. 93][94] The Military Academy of Medical Sciences of the People’s Republic of China claimed to have “successfully” developed the recombinant coronavirus vaccine, and said it is preparing for “large-scale” production, according to a statement issued by the Chinese Ministry of Defense. 95] The Coalition for Innovations in Epidemic Preparedness (CEPI) is funding three vaccine projects[96] and hopes to have a vaccine in trials by June 2020 and approved and ready in one year. The University of Queensland in Australia received US$10.6 million in funding from CEPI to develop a “molecular clamp” vaccine platform. 97][98][99] Moderna Inc. is developing an mRNA vaccine with funding from ECCE. 100][101] Inovio Pharmaceuticals received a grant from CEPI and designed a vaccine within two hours of receiving the gene sequence. 102] The vaccine is being manufactured so that it can first be tested on animals. [102]
Israeli scientists hope to have an oral vaccine ready in 90 days, after going through the safety testing phase. [103]
By early March 2020, some 30 vaccine candidates were in development, with Gilead Sciences and Ascletis Pharma products in Phase III clinical trials. [104][105]
On Jan. 23, Gilead Sciences was in communication with researchers and physicians in the United States and China about the ongoing outbreak of Wuhan coronavirus and the potential use of Remdesivir as an investigational treatment. [106]
In late January 2020, Chinese medical researchers expressed their intention to begin clinical trials with remdesivir, chloroquine and lopinavir/ritonavir, which appeared to have inhibitory effects on SARS-CoV-2 at the cellular level in exploratory in vitro experiments. 107] Nitazoxanide has been recommended for further in vivo studies after demonstrating low-concentration inhibition of SARS-CoV-2. [108] On February 2, 2020, physicians in Thailand claimed to have successfully treated a patient with a combination of lopinavir/ritonavir and the influenza drug oseltamivir. 109][110] On February 5, China began patenting the use of remdesivir against the disease. 111][112][113][114][115][116] Phase 3 clinical trials with remdesivir are under way in March in the United States, China, and Italy. [117][118][119]
In late January, the Russian Ministry of Health identified three adult medications that could help treat the disease. They are ribavirin, lopinavir/ritonavir and beta-1b interferon. These drugs are commonly used to treat hepatitis C, HIV infection and multiple sclerosis, respectively. The ministry provided Russian hospitals with descriptions and guidelines on the mechanism of action of treatment and recommended doses. 120] In February, China began using triazavirin, a 2014 drug developed in Russia, to test its effectiveness in controlling the disease. This drug was created at Ural Federal University in Ekaterinburg to treat H5N1 (bird) flu. It has been used against COVID-19 because of the similarity between the two diseases. The drug also appears to be effective against Rift Valley Fever and West Nile virus, among others. [121][122]
On March 18 an article reports that lopinavir/ritonavir treatment is negative in clinical trials with 199 patients in China. There are no benefits. [123]
Chinese researchers discovered that Arbidol, an antiviral drug used to treat the flu, could be combined with Darunavir, a drug used in the treatment of HIV, to treat patients with coronaviruses. [124][125]
Chloroquine phosphate has shown apparent efficacy in the treatment of VID-19-associated pneumonia. In clinical trials with 100 patients it was found to be superior to control treatment in inhibiting exacerbation of pneumonia, improving pulmonary imaging findings, promoting negative conversion to the virus, and shortening disease. Research results showed that the SARS-CoV-2 protein ORF8 and the surface glycoprotein could bind to porphyrin, respectively, while the SARS-CoV-2 proteins orf1ab, ORF10 and ORF3a could coordinate with heme to dissociate iron to form porphyrin. The mechanism seriously interfered with the normal anabolic pathway of heme in the human body and this results in human disease. Based on validation analysis of these findings, chloroquine may prevent orf1ab, ORF3a and ORF10 from attacking heme to form porphyrin, and inhibit ORF8 and surface glycoprotein binding to porphyrins to some extent. [126][127][128]
Researchers at the Norwegian University of Science and Technology (NTNU) have created a database with 120 broad-spectrum antiviral agents that are safe for humans and identified 31 drug candidates for the treatment of SARS-CoV-2
China’s National Center for Biotechnology Development said March 17 that the antiviral Favipiravir, an RNA polymerase inhibitor,[130] showed positive results in a case-control study of 80 patients at Shenzhen People’s Hospital No. 3, those treated with Favipiravir tested negative within a shorter period of time compared to those in the control group, and recommended that it be included in treatment. [131][132]
Recent studies have shown that initial priming of the peak protein by the transmembrane protease serine 2 (TMPRSS2) is essential for the entry of SARS-CoV-2, SARS-CoV and MERS-CoV through interaction with the ACE2 receptor.133][134] These findings suggest that the TMPRSS2 inhibitor Camostat, approved for clinical use in Japan to inhibit fibrosis in liver and kidney disease, postoperative reflux esophagitis, and pancreatitis, may be an effective off-label treatment option.
Hydroxychloroquine, a less toxic chloroquine derivative, would be more potent in inhibiting SARS-CoV-2 infection in vitro. 135][136] On March 16, 2020, a leading French authority and advisor to the French government on COVID-19, Professor Didier Raoult of the University Hospital Institute of Infectious Diseases (IHU-Méditerranée infection) in Marseille (Bouches-du-Rhône, Provence-Alpes-Côte d ‘Azur), announced that a trial involving 24 patients in southeastern France had shown chloroquine to be an effective treatment for COVID-19.137][138] These patients were given 600 mg of hydroxychloroquine (brand name Plaquenil) every day for 10 days. This led to a “rapid and effective acceleration of their healing process, and a sharp decrease in the amount of time they remained contagious. 139] Although chloroquine has a long history of safety, patients were closely monitored for drug interactions and possible serious side effects. Professor Raoult said, “We included everyone who agreed [to be treated], which was almost everyone. Two cities in the protocol, Nice and Avignon, gave us patients [infected] who had not yet received treatment … We were able to determine that the patients who had not received Plaquenil [the drug containing hydroxychloroquine] were still contagious after six days, but of those who had received Plaquenil, after six days, only 25% were still contagious. 140] In Australia, the Director of the Clinical Research Centre at the University of Queensland, Professor David Paterson, announced his intention to conduct a major clinical research trial on the efficacy of chloroquine and remedesivir as treatments for COVID-19. Professor Paterson hoped to begin enrolling patients by the end of March 2020. [142]
A limited French study shows that hydroxychloroquine combined with azithromycin is faster than hydroxychloroquine alone in transforming VOC-19 patients to negative. [143][144]
Against the cytokine storm
Tocilizumab has been included in treatment guidelines by the National Health Commission of China after a small study was completed. 145][146] It is undergoing national phase 2 nonrandomized testing in Italy after showing positive results in people with severe disease. 147][148][149] In combination with a serum ferritin blood test to identify cytokine storms, it is intended to counteract such developments, which are believed to be the cause of death in some affected individuals. 150][151] The interleukin-6 receptor antagonist was approved by the FDA for the treatment of cytokine release syndrome induced by a different cause, CAR T cell therapy, in 2017
Northwell Health’s Feinstein Institute announced in March a study of “a human antibody that can prevent activity” of IL-6. Called sarilumab, it was developed jointly by Regeneron Pharmaceuticals and Sanofi. [153]
Passive antibody therapy
Research is being conducted into the use of blood donations from healthy people who have already recovered from COVID-19, [154] a strategy that has also been tested for SARS, a former cousin of COVID-19. 154] Other forms of passive antibody therapy, such as manufactured monoclonal antibodies, may come after biopharmaceutical development,[154] but production of convalescent serum could be increased for faster deployment. [155]
San Francisco-based Vir Biotechnology is evaluating the effectiveness of previously identified monoclonal antibodies (mAbs) against the virus. [156]
Researchers from Utrecht University and Erasmus MC announced that they have found a human monoclonal antibody that blocks SARS-CoV-2 infection
Of the first 41 cases of VIDOC-19 that were treated in Wuhan hospitals, thirteen (32 %) required intensive care and six (15 %) died. 56] Many of those who died had comorbidities such as high blood pressure, diabetes, or cardiovascular disease that weakened their immune systems. [159]
In these early cases that ended in death, the median duration of illness was 14 days and the total range was 6 to 41 days. [160]
Of the confirmed cases, 80.9% were classified as mild. [161][162]
As of 20 February 2020, 2 114 of the 55 924 laboratory-confirmed cases had died, representing a crude fatality ratio of 3,8 %. The overall CFR varied at that point in the epidemic, depending on the location and intensity of transmission, from 5.8% in Wuhan to 0.7% in other areas in China. In China, the overall CFR was higher in the early stages of the outbreak (17.3% for cases with onset of symptoms from 1-10 January) and has been reduced over time to 0.7% for patients with onset of symptoms after 1 February. The level of care has evolved over the course of the outbreak. Mortality increases with age and appears to be higher among men than women (4.7% vs. 2.8%). While patients who did not report comorbid conditions had a CFR of 1.4%, patients with comorbidity died at much higher rates: 13.2% for those with cardiovascular disease, 9.2% for diabetes, 8.4% for hypertension, 8.0% for chronic respiratory disease and 7.6% for cancer. [163]
As of 3 March 2020 globally, 3,110 of the 90,892 reported cases of VOC-19 had died (3.4%), according to figures provided by the WHO Director. [164] [165]
In a study published online in the journal Pediatrics, researchers analyzed 2,143 cases of children under the age of 18 who were reported to the CCDC as of February 8, 2020. About half of the children had mild symptoms, such as fever, fatigue, cough, congestion and possibly nausea or diarrhea. About 39% became moderately ill, with additional symptoms including pneumonia or lung problems revealed by the CT scan, but no obvious respiratory distress. About 4% had no symptoms at all. But 125 children, nearly 6%, developed a very serious illness and one 14-year-old boy with a confirmed coronavirus infection died. Thirteen of them were considered “critical”, on the verge of respiratory or organic failure. The others were classified as “severe” because they had serious respiratory problems. More than 60% of the 125 children who became seriously ill or had a critical illness were 5 years old or younger. Forty of them were infants, under 12 months old. [166]
Adjusted fatality rate
Mortality rate among symptomatic infections
| Age of patients
(in years) |
Mortality
(in %) |
|---|---|
| 0–9 | 0,019% |
| 10–19 | 0,046% |
| 20–29 | 0,19% |
| 30–39 | 0,38% |
| 40–49 | 0,82% |
| 50–59 | 2,7% |
| 60–69 | 9,4% |
| 70–79 | 20% |
| 80 or more | 36% |
* Adjusted for late mortality and adjusted for unidentified symptomatic cases.
Mortality rate among all symptomatic and asymptomatic infections.
| Age of patients
(in years) |
Mortality
(in %) |
|---|---|
| 0–9 | 0,022% |
| 10–19 | 0,091% |
| 20–29 | 0,18% |
| 30–39 | 0,4% |
| 40–49 | 0,82% |
| 50–59 | 1,3% |
| 60–69 | 4,6% |
| 70–79 | 9,8% |
| 80 or more | 18% |
Refː [149]
Until 11 February 2020 in China, the probability of death among people infected with symptoms is estimated at 3.3% (2.9-3.8), with a sharp increase from over 60 years to 36% over 80 years. This is specific to the situation in Hubei, China, during this period. 149] Of a total of 72,314 patient records, 44,672 (61.8%) were confirmed cases. A total of 1 023 deaths were recorded among the confirmed cases, with a fatality rate of 2.3%. [161]
Korea is the only country in the world where systematic analyses of large population groups were carried out (about 10 000 per day, with 210 000 counted up to 10 March). [167]
Some international organizations, such as the WHO, have published preventive measures to reduce the transmission of the virus. They are similar to those that have been recommended to prevent infection by other coronaviruses and include
- Frequent hand washing with soap and water.
- When coughing or sneezing, cover your mouth and nose with the ulnar cavity (the concavity that forms the inside of the arm when bent at the elbow).
- Keep at least one meter away from other people, “particularly those who cough, sneeze and have a fever.
- Avoid touching your eyes, nose and mouth.
- Go to the doctor in case of fever, cough and difficulty to breathe, calling in advance if you are in areas where the virus is spreading or if you have been visited in the last 14 days.
- Stay home if you begin to feel sick, even with mild symptoms such as headache and mild rhinorrhea, until you recover if you are in areas where the virus is spreading or if you have visited them in the last 14 days. [168]
To reduce the chances of infection, health organizations recommend avoiding close contact with sick people; washing hands frequently with soap and water; not touching eyes, nose, or mouth with unwashed hands; and practicing good respiratory hygiene. [169][170]
People who are already infected are advised to stay home, except to receive medical care, call ahead before visiting a health care provider, wear a face mask (especially in public), cover coughs and sneezes with a tissue, wash hands regularly with soap and water, and avoid sharing personal items in the home. 171] Depending on the legislation of each country, the intentional transmission of the virus is punishable according to the legal system where the event occurs. [172]
There is currently no vaccine. [173]
Several governments discourage all non-essential travel to countries and areas affected by the outbreak. 174] The Hong Kong government warned anyone traveling outside the city not to touch animals; not to eat bushmeat; and to avoid visiting wet markets, live bird markets, and farms. 175] There is no evidence that pets, such as dogs and cats, can be infected. 176] The Chinese government has banned the trade and consumption of wild animals. [177]
For health care providers caring for someone who may be infected, standard precautions, contact precautions, and airborne precautions with eye protection are recommended. [178]
Hand washing
Hand washing is recommended to prevent the spread of coronavirus. The CDC recommends:[179]
- Wash your hands often with soap and water for at least 20 seconds, especially after going to the bathroom; before eating; and after blowing your nose, coughing or sneezing.
- If soap and water are not available, use an alcohol-based hand sanitizer with at least 60% alcohol. Always wash your hands with soap and water if your hands are visibly dirty.
People should avoid touching their eyes, nose or mouth with unwashed hands. [180]
Coronaviruses can survive and remain contagious on inanimate surfaces such as metal, glass, or plastic for up to nine days. Methods to remove the virus from surfaces include chlorine-based disinfectants, 75% ethanol, peracetic acid, and chloroform. [176]
Respiratory hygiene

People wearing surgical masks in Guangzhou.
Poster on ‘Face Hair Styles and Mask Respirators’ published by the US Centers for Disease Control
Health organizations recommend covering your mouth and nose with your elbow when you cough or sneeze or covering your mouth and nose with a tissue (which should then be disposed of immediately) and then washing your hands with an alcohol-based hand sanitizer or soap and water. [181][182]
Those who suspect they are infected should wear a surgical mask (especially when in public) and call a doctor for medical advice. 175][183][184] By limiting the volume and travel distance of dispersed expiratory droplets when speaking, sneezing, and coughing, masks may benefit public health by reducing transmission from those infected unknowingly. [185]
If a mask is not available, anyone experiencing respiratory symptoms should cover themselves with a tissue when coughing or sneezing, quickly dispose of it in the trash, and wash their hands. If a tissue is not available, people can cover their mouth or nose with a bent elbow. [180]
Masks are also recommended for those caring for someone who may have the disease. 184]Rinsing the nose, gargle with mouthwash, and eating garlic are not effective methods. [176]
The WHO advises the following best practices for mask use: [184]
- Place the mask carefully to cover the mouth and nose and tie it securely to minimize any gaps between the face and the mask; while in use, avoid touching the mask;
- Remove the mask using proper technique (i.e., do not touch the front but remove the lace from the back);
- After removal or whenever you inadvertently touch a used mask, clean your hands with an alcohol or soap and water-based hand sanitizer if visibly soiled;
- Replace masks with a new, clean, dry mask as soon as they are wet;
- Do not reuse single-use masks; discard single-use masks after each use and dispose of them immediately after removal.
Healthcare professionals who interact directly with people who have the disease are advised to use respirators that are at least as protective as NIOSH-certified N95, the EU FFP2 standard, or equivalent, in addition to other personal protective equipment. [184][186]
There is no evidence that masks protect uninfected people at low risk, and wearing them can create a false sense of security. Surgical masks are widely used by healthy people in Hong Kong, [187] Japan, [188] Singapore [189] [190] and Malaysia. [191]
Self-isolation
In addition to the above guidance on hand washing and respiratory hygiene, public health agencies advise that sick people who suspect they may have VOC-19 should restrict activities outside the home, except to obtain medical care: [192] [193] [194]
- No going to work, school or public areas. Avoiding the use of public transportation, ride sharing or taxis
- Call in advance before visiting a doctor.
- Separate yourself from other people and animals in the home; do not share personal items; use a separate bathroom if available
- Use a household cleaner to clean all frequently touched surfaces (counters, toilets, doorknobs, etc.) every day
Prevention measures recommended by WHO include regular hand washing with soap and water, covering the mouth and nose with a bendable elbow when coughing or sneezing, and avoiding direct contact with people showing symptoms of respiratory disease or with live animals without adequate protective measures. [195][196][197]

People stand in line in front of a supermarket.
Social distancing includes infection control actions designed to slow the spread of disease by minimizing close contact between people. Methods include quarantines, travel restrictions, and closures of schools, workplaces, stadiums, theaters, or shopping centers. People can also apply methods of social distancing by limiting travel, avoiding crowded areas, and staying away from sick people. 198][199] Many governments now require or recommend social distancing in regions affected by the outbreak. [200][201][202]
Older adults and those with serious chronic conditions face an increased risk of serious illness and complications from COVID-19, and the U.S. CDC has advised them to avoid crowds and stay home as much as possible in community outbreak areas. [203]
Some countries, such as Canada or the United States, issued guidelines for not shaking hands, hugging or kissing. 204] Some countries such as India have advised their citizens to avoid spitting in public places. 205] The World Health Organization (WHO) now recommends for the general population to maintain “at least 1 meter (3 feet) of distance between you and other people, particularly those who cough, sneeze, and have a fever
Quarantines, restrictions on human trafficking and the isolation that is taking place because of the pandemic have been shown to have negative psychological effects. 206] At the end of January, the Chinese National Health Commission published a guide to managing psychological crises, advocating for the intervention of affected persons, close contacts, those locked in their homes, patients’ families and friends, health workers, and the general public as needed. [207][208]
Sources: References
- ↑
- ↑.”New Coronavirus Disease, COVID-19. Information for the public. What can I do to protect myself from the new coronavirus and other respiratory viruses? Ministry of Health (Spain). Archived from the original March 10, 2020. Consulted on March 4, 2020.
- ↑ a b c d“Coronavirus, writing keys.” Fundéu BBVA. 29th January 2020. Archived from the original on 20 March 2020. Consulted on 20 March 2020.
- ↑ World Health Organization, ed. “Statement by the Director-General of WHO at the 2019-nCoV press conference on 11 February 2020”. who.int. Archived since original 20 February 2020. Accessed on 11 February 2020.
- ↑”国家卫生健康委关于新型冠状病毒肺炎暂命名事宜的通知” (in Chinese). February 7, 2020. Filed since original February 16, 2020. Accessed on 11 February 2020.
- ↑ “Coronavirus infections”. Medline Plus (U.S. National Library of Medicine). Archived since original March 5, 2020. Accessed March 4, 2020. “Many of the people with COVID-19 have pneumonia in both lungs. …] Usually [coronaviruses] cause mild to moderate upper respiratory infections, such as the common cold. But they can also cause more serious illnesses, such as bronchitis and pneumonia
- ↑ “What is known about the coronavirus? Symptoms, diagnosis, lethality…” El Pais. February 27th, 2020. Archived from the original February 28, 2020. “The virus infects the respiratory tract and causes symptoms ranging from mild (dry cough, fever…) to acute respiratory failure and life-threatening pneumonias. The associated disease has been named Covid-19.”
- ↑ “What are the differences between the coronavirus and a flu?” The New York Times. March 2, 2020. Archived from original March 3, 2020. “Pneumonia is common among coronavirus patients, even among those whose cases are not severe.”
- ↑ a b c Gorbalenya, A. E.; Baker, S. C.; Baric, R. S.; de Groot, R. J.; Drosten, C.; Gulyaeva, A. A.; Haagmans, B. L.; Lauber, C.; Leontovich, A. M.; Neuman, B. M.; Penzar, D.; Poon, L. L. M.; Samborskiy, D.; Sidorov, I. A.; Sola, I.; Ziebuhr, J. “Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group”. bioRxiv. doi:10.1101/2020.02.07.937862. Archived since original 11 February 2020. Accessed February 11, 2020.
- ↑ “Coronavirus disease named Covid-19”. BBC News. February 11, 2020. Archived since original Feb. 11, 2020. Accessed February 11, 2020.
- ↑ a b c Hui, D. S.; Azhar, E. I.; Madani, T. A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T. D.; Memish, Z. A.; Drosten, C.; Zumla, A.; Petersen, E. (14 January 2020). “The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – The latest 2019 novel coronavirus outbreak in Wuhan, China. International Journal of Infectious Diseases: 264-266. PMID 31953166. doi:10.1016/j.ijid.2020.01.009.
- ↑ World Health Organization (WHO) (ed.). “Q&A on coronaviruses. Archived since original 20 January 2020. Accessed 27 January 2020.
- ↑ a b World Health Organization, ed. “Opening address by the Director-General of WHO at the press conference on COVID-19 on 11 March 2020”. Archived from the original March 12, 2020. Accessed on 11 March 2020.
- ↑ a b c European Centre for Disease Prevention and Control (ECDC) (ed.) “Q & A on novel coronavirus. Archived since original 11 February 2020. Accessed 11 February 2020.
- ↑ a b Australian Government Department of Health, ed. “Novel coronavirus (2019-nCoV)”. Archived since original 9 February 2020. Accessed 11 February 2020.
- ↑ “Sudden loss of smell, possible symptom of coronavirus”. La Vanguardia. March 23rd, 2020. Archived from the original March 23, 2020. Consulted on March 25, 2020.
- ↑. Centers for Disease Control and Prevention (USA), ed. “Coronavirus Disease 2019 (COVID-19). Archived from original March 25, 2020. Accessed 23 March 2020.
- ↑. “Sepsis causes most coronavirus deaths. El Mundo (Spain). March 24, 2020. Archived since original March 24, 2020. Consulted on March 24, 2020.
- ↑ Álef Libera el Conocimiento, ed. “Carl Flügge and the Spittlebug Speech”. Archived from the original September 13, 2016. Accessed 22 March 2020.
- ↑ Peng, Xian; Xu, Xin; Li, Yuqing; Cheng, Lei; Zhou, Xuedong; Ren, Biao (3 March 2020). “Transmission routes of 2019-nCoV and controls in dental practice”. International Journal of Oral Science 12 (1): 1-6. ISSN 2049-3169. doi:10.1038/s41368-020-0075-9. Accessed 9 March 2020.
- ↑ Centers for Disease Control and Prevention, USA, ed. “Symptoms of Novel Coronavirus (2019-nCoV). www.cdc.gov Archived since original March 24, 2020. Accessed February 10, 2020.
- ↑ Centers for Disease Control and Prevention, USA, ed. “Symptoms. www.cdc.gov Filed since original January 30, 2020. Accessed February 11, 2020.
- ↑ Lai, C. C.; Shih, T. P.; Ko, W. C.; Tang, H. J.; Hsueh, P. R. (February 2020). “Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges”. International Journal of Antimicrobial Agents: 105924. PMID 32081636. doi:10.1016/j.ijantimicag.2020.105924.
- ↑ Velavan, Thirumalaisamy P.; Meyer, Christian G. (12 February 2020). “The COVID-19 epidemic. Tropical Medicine & International Health 25 (3): 278-280. ISSN 1365-3156. PMID 32052514. doi:10.1111/tmi.13383.
- ↑ “Do you need to wear a mask to protect yourself from the coronavirus? The Feed. Archived since original 30 January 2020. Accessed 11 February 2020.
- ↑ “Novel coronavirus named ‘Covid-19’: WHO. Today. Archived since original 21 March 2020. Accessed 11 February 2020.
- ↑ World Health Organization, ed. “New coronavirus – China”. www.who.int. Archived since original 21 January 2020. Accessed 27 January 2020.
- ↑ World Health Organization (ed.). “Statement on the second meeting of the International Health Regulations Emergency Committee (2005) on the outbreak of the new coronavirus (2019 nCoV)”. www.who.int. Archived from original 20 February 2020. Accessed 11 February 2020.
- ↑. Johns Hopkins University, ed. “Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering”. Archived from original 27 March 2020. Consulted on March 27, 2020.
- ↑ Zafra, M.; Sevillano Pires, L.; Galocha, A.; Blanco, P. R. “Casos confirmados en España y en el mundo y claves para entender el coronavirus. The map of the coronavirus”. El País (Spain). Archived from the original March 27, 2020. Consulted on 27th March 2020.
- ↑ Johns Hopkins University (ed.). “Coronavirus COVID-19 Global Cases”. Consulted on March 20, 2020.
- ↑ “Coronavirus Update (Live): 307,627 Cases and 13,050 Deaths from COVID-19 Virus Outbreak-Worldometer”. www.worldometers.info.
- ↑ “Here Comes the Coronavirus Pandemic: Now, after many fire drills, the world may be facing a real fire”. The New York Times. February 29, 2020. Accessed 1 March 2020.
- ↑ Perper, Rosie (5 March 2020). “As the coronavirus spreads, one study predicts that even the best-case scenario is 15 million dead and a $2.4 trillion hit to global GDP. Business Insider. Archived since original March 5, 2020. Accessed March 5, 2020.
- ↑ Clamp, Rachel (5 March 2020). “Coronavirus and the Black Death: spread of misinformation and xenophobia shows we haven’t learned from our past”. The Conversation. Archived since original March 6, 2020. Accessed 14 March 2020.
- ↑ Tavernise, Sabrina; Oppel Jr, Richard A. (March 23, 2020). “Spit On, Yelled At, Attacked: Chinese-Americans Fear for Their Safety. The New York Times. Accessed March 23, 2020.
- ↑. “Coronavirus Disease 2019 (COVID-19). Answers to the most frequently asked questions”. Centers for Disease Control and Prevention, USA. Archived since original March 26, 2020.
- ↑ World Health Organization, ed. “WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020”. who.int Archived since original 12 February 2020. Accessed on 11 February 2020.
- ↑ Zhou, Peng; Yang, Xing-Lou; Wang, Xian-Guang; Hu, Ben; Zhang, Lei; Zhang, Wei; Si, Hao-Rui; Zhu, Yan; Li, Bei; Huang, Chao-Lin; Chen, Hui-Dong; Chen, Jing; Luo, Yun; Guo, Hua; Jiang, Ren-Di; Liu, Mei-Qin; Chen, Ying; Shen, Xu-Rui; Wang, Xi; Zheng, Xiao-Shuang; Zhao, Kai; Chen, Quan-Jiao; Deng, Fei; Liu, Lin-Lin; Yan, Bing; Zhan, Fa-Xian; Wang, Yan-Yi; Xiao, Gengfu; Shi, Zheng-Li (23 January 2020) “Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin”. bioRxiv: 2020.01.22.914952. doi:10.1101/2020.01.22.914952. Archived since original 24 January 2020. Accessed on 5 February 2020.
- ↑”Surveillance case definitions for human infection with novel coronavirus (nCoV)”
- ↑”Novel coronavirus (2019-nCoV), Wuhan, China”. Cdc.gov. January 10, 2020. Accessed January 16, 2020.
- ↑ Zhang, Y.-Z. (12 January 2020). Wuhan seafood market pneumonia virus isolate Wuhan-Hu-1, complete genome. Bethesda MD. p. GenBank. Accessed January 13, 2020.
- ↑ “中国疾病预防控制中心”. www.chinacdc.cn. Accessed 9 January 2020.
- ↑ “New-type coronavirus causes pneumonia in Wuhan: expert – Xinhua | English.news.cn”. www.xinhuanet.com. Accessed 9 January 2020.
- ↑ “CoV2020”. platform.gisaid.org. Accessed 12 January 2020.
- ↑. “This is what happens when the Coronavirus attacks your body. El Pais. 22/03/2020. p. www.eldiario.es.
- ↑ World Health Organization (2020), Novel Coronavirus (2019-nCoV): situation report, 6, World Health Organization
- ↑ “Symptoms of Novel Coronavirus (2019-nCoV)”. Centers for Disease Control and Prevention. January 31, 2020. Filed since original January 30, 2020. Accessed February 2, 2020.
- ↑ “WHO COVID-19 situation report 29”. World Health Organization. 19 February 2020.
- ↑ “Q&A on coronaviruses (COVID-19): How long is the incubation period for COVID-19?”. www.who.int Accessed 25 February 2020.
- ↑ hermesauto (11 February 2020). “Coronavirus: New study finds incubation period of up to 24 days”. The Straits Times. Archived from original 11 February 2020. Accessed 12 February 2020.
- ↑. “Coronavirus incubation could be as long as 27 days, Chinese provincial government says”. Reuters. 22 February 2020.
- ↑. Chen, Nanshan; Zhou, Min; Dong, Xuan; Qu, Jieming; Gong, Fengyun; Han, Yang; Qiu, Yang; Wang, Jingli; Liu, Ying; Wei, Yuan; Xia, Jia’an (30 January 2020). “Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 0. ISSN 0140-6736. PMID 32007143. doi:10.1016/S0140-6736(20)30211-7.
- ↑ Hessen, Margaret Trexler (27 January 2020). “Novel Coronavirus Information Center: Expert guidance and commentary”. Elsevier Connect. Archived since original 30 January 2020. Accessed January 31, 2020.
- ↑ “Coronavirus About Symptoms and Diagnosis”. Centers for Disease Control and Prevention. United States. January 30, 2020. Filed since original January 30, 2020. Accessed February 1, 2020.
- ↑ a b Huang, Chaolin; Wang, Yeming; Li, Xingwang; Ren, Lili; Zhao, Jianping; Hu, Yi; Zhang, Li; Fan, Guohui; Xu, Jiuyang; Gu, Xiaoying; Cheng, Zhenshun (24 January 2020) “Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. ISSN 0140-6736. PMID 31986264. doi:10.1016/S0140-6736(20)30183-5.
- ↑ a b World Health Organization. “Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)”. pp. 11-12. Accessed 11 March 2020.
- ↑ Hui, David S.; Azhar, Esam EI; Madani, Tariq A.; Ntoumi, Francine; Kock, Richard; Dar, Osman; Ippolito, Giuseppe; Mchugh, Timothy D. et al. “The continuing epidemic threat of novel coronaviruses to global health – the latest novel coronavirus outbreak in Wuhan, China. International Journal of Infectious Diseases 0 (0). ISSN 1201-9712. doi:10.1016/j.ijid.2020.01.009.
- ↑. “Experts explain the latest bulletin of unknown cause of viral pneumonia”. Wuhan Municipal Health Commission. 11 January 2020. Accessed 11 January 2020.
- ↑ Schnirring, Lisa (6 January 2020). “Questions still swirl over China’s unexplained pneumonia outbreak”. CIDRAP. Accessed 7 January 2020.
- ↑ “Pneumonia of Unknown Cause in China – Watch – Level 1, Practice Usual Precautions – Travel Health Notices | Travelers’ Health | CDC”. wwwnc.cdc.gov. 6 January 2020. Accessed January 7, 2020.
- ↑ Chaolin Huang, MD * et, al (January 20, 2020). “Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. doi:10.1016/S0140-6736(20)30183-5. Accessed 25 January 2020.
- ↑ Chen Wang et al (24 January 2020). “A novel coronavirus outbreak of global health concern. The Lancet. doi:10.1016/S0140-6736(20)30185-9. Accessed January 25, 2020.
- ↑ Pan, Xingfei; Chen, Dexiong; Xia, Yong; Wu, Xinwei; Li, Tangsheng; Ou, Xueting; Zhou, Liyang; Liu, Jing (19 February 2020). “Asymptomatic cases in a family cluster with SARS-CoV-2 infection”. The Lancet Infectious Diseases 0. ISSN 1473-3099. PMID 32087116. doi:10.1016/S1473-3099(20)30114-6.
- ↑ “Q&A on coronaviruses”. who.int. Archived since original January 20, 2020. Consulted on January 27, 2020.
- ↑ Liu, Jia; Cao, Ruiyuan; Xu, Mingyue; Wang, Xi; Zhang, Huanyu; Hu, Hengrui; Li, Yufeng; Hu, Zhihong et al. “Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro”. Cell Discovery 6 (1): 1-4. ISSN 2056-5968. doi:10.1038/s41421-020-0156-0. Accessed March 22, 2020.
- ↑ Jin, Ying-Hui; Cai, Lin; Cheng, Zhen-Shun; Cheng, Hong; Deng, Tong; Fan, Yi-Pin; Fang, Cheng; Huang, Di; Huang, Lu-Qi; Huang, Qiao; Han, Yong (6 February 2020). “A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version)”. Military Medical Research 7 (1): 4. ISSN 2054-9369. PMID 32029004. doi:10.1186/s40779-020-0233-6.
- ↑ Schirring, Lisa; 2020 (January 16, 2020). “Japan has 1st novel coronavirus case; China reports another death. CIDRAP. Archived since original 20 January 2020. Accessed January 16, 2020.
- ↑ “Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases: Interim guidance”. World Health Organization. Archived since original 20 January 2020. Accessed 28 January 2020.
- ↑ “2019 Novel Coronavirus (2019-nCoV) Situation Summary”. Centers for Disease Control and Prevention. January 30, 2020. Archived from original January 26, 2020. Accessed January 30, 2020.
- ↑ “Real-Time RT-PCR Panel for Detection 2019-nCoV”. Centers for Disease Control and Prevention. January 29, 2020. Archived from original January 30, 2020. Accessed February 1, 2020.
- ↑ Brueck, Hilary (30 January 2020). “There’s only one way to know if you have the coronavirus, and it involves machines full of spit and mucus”. Business Insider. Archived since original 1 February 2020. Accessed 1 February 2020.
- ↑ “Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe”. GlobeNewswire News Room. 30 January 2020. Archived since original January 31, 2020. Accessed February 1, 2020.
- ↑ “Undiagnosed pneumonia – China (HU) (01): wildlife sales, market closed, RFI Archive Number: 20200102.6866757”. Pro-MED-mail. International Society for Infectious Diseases. Archived since original 22 January 2020. Accessed January 13, 2020.
- ↑ Cohen, Jon; Normile, Dennis (17 January 2020). “New SARS-like virus in China triggers alarm. Science 367 (6475): 234-235. ISSN 0036-8075. PMID 31949058. doi:10.1126/science.367.6475.234.
- ↑ Parry, Jane (January 2020). “China coronavirus: cases emerge as official admits human to human transmission”. British Medical Journal 368: m236. ISSN 1756-1833. PMID 31959587. doi:10.1136/bmj.m236.
- ↑ Voytko, Lisette. “WHO Declares Coronavirus A Global Health Emergency, Praises China’s ‘Extraordinary Measures'”. Forbes. Filed since original February 1, 2020. Accessed February 1, 2020.
- ↑ “Undiagnosed pneumonia – China (HU) (01): wildlife sales, market closed, RFI Archive Number: 20200102.6866757”. Pro-MED-mail. International Society for Infectious Diseases. Archived since original 22 January 2020. Accessed January 13, 2020.
- ↑ Hui, David S.; Azhar, Esam EI; Madani, Tariq A.; Ntoumi, Francine; Kock, Richard; Dar, Osman; Ippolito, Giuseppe; Mchugh, Timothy D.; Memish, Ziad A.; Drosten, Christian; Zumla, Alimuddin (14 January 2020). “The continuing epidemic threat of novel coronaviruses to global health – the latest novel coronavirus outbreak in Wuhan, China. International Journal of Infectious Diseases 91: 264-266. ISSN 1201-9712. PMID 31953166. doi:10.1016/j.ijid.2020.01.009. Archived since original 31 January 2020. Accessed on January 16, 2020.
- ↑ Cohen, Jon; Normile, Dennis (17 January 2020). “New SARS-like virus in China triggers alarm”. Science 367 (6475): 234-235. ISSN 0036-8075. PMID 31949058. doi:10.1126/science.367.6475.234.
- ↑ Parry, Jane (January 2020). “China coronavirus: cases emerge as official admits human to human transmission”. British Medical Journal 368: m236. ISSN 1756-1833. PMID 31959587. doi:10.1136/bmj.m236.
- ↑ Wiles, Siouxsie (9 March 2020). “The three phases of Covid-19 – and how we can make it manageable”. The Spinoff. Accessed 9 March 2020.
- ↑ Anderson, Roy M; Heesterbeek, Hans; Klinkenberg, Don; Hollingsworth, T Déirdre (March 2020). “How will country-based mitigation measures influence the course of the COVID-19 epidemic? The Lancet. doi:10.1016/S0140-6736(20)30567-5. “A key issue for epidemiologists is helping policy makers decide the main objectives of mitigation-e.g., minimising morbidity and associated mortality, avoiding an epidemic peak that overwhelms health-care services, keeping the effects on the economy within manageable levels, and flattening the epidemic curve to wait for vaccine development and manufacture on scale and antiviral drug therapies
- ↑ Nebehay, Stephanie; Kelland, Kate; Liu, Roxanne (5 February 2020). “WHO: ‘no known effective’ treatments for new coronavirus”. Thomson Reuters. Archived since original Feb. 5, 2020. Accessed 5 February 2020.
- ↑ Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 28 January 2020. doi 10.5582/bst.2020.01020
- ↑ “China CDC developing novel coronavirus vaccine”. Xinhua. January 26, 2020. Archived from original January 26, 2020. Accessed January 28, 2020.
- ↑ Holshue, Michelle L.; DeBolt, Chas; Lindquist, Scott; Lofy, Kathy H.; Wiesman, John; Bruce, Hollianne; Spitters, Christopher; Ericson, Keith; Wilkerson, Sara; Tural, Ahmet; Diaz, George (31 January 2020). “First Case of 2019 Novel Coronavirus in the United States”. New England Journal of Medicine: NEJMoa2001191. ISSN 0028-4793. PMID 32004427. doi:10.1056/NEJMoa2001191.
- ↑ “Anti-novel coronavirus drug under clinical trial: official”. Xinhuanet. Archived since original February 3, 2020. Accessed February 3, 2020.
- ↑ Xu, Zhijian; Peng, Cheng; Shi, Yulong; Zhu, Zhengdan; Mu, Kaijie; Wang, Xiaoyu; Zhu, Weiliang (28 January 2020). “Nelfinavir was predicted to be a potential inhibitor of 2019 nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation”. bioRxiv: 2020.01.27.921627. doi:10.1101/2020.01.27.921627 – via www.biorxiv.org.
- ↑ a b Paules, Catharine I.; Marston, Hilary D.; Fauci, Anthony S. (January 23, 2020). “Coronavirus Infections-More Than Just the Common Cold. JAMA. PMID 31971553. doi:10.1001/jama.2020.0757.
- ↑ Chen, Wen-Hsiang; Strych, Ulrich; Hotez, Peter J; Bottazzi, Maria Elena (3 March 2020). “The SARS-CoV-2 Vaccine Pipeline: an Overview”. Current Tropical Medicine Reports. doi:10.1007/s40475-020-00201-6.
- ↑ “With Wuhan virus genetic code in hand, scientists begin work on a vaccine”. Reuters. January 24, 2020. Reuters. 24 January 2020.
- ↑ “China CDC developing novel coronavirus vaccine”. Xinhua. January 26, 2020.
- ↑ “Chinese scientists race to develop vaccine as coronavirus death toll jumps”. SCMP. January 26, 2020.
- ↑. From 2020, March 17. “China claims to have “successfully” developed the coronavirus vaccine and is preparing for large-scale production”. Infobae. Accessed March 18, 2020.
- ↑ hermesauto (January 23, 2020). “Wuhan virus: Work to start on three possible vaccines, says epidemic response group”. The Straits Times. Accessed 26 January 2020.
- ↑ “CEPI to fund three programmes to develop vaccines against the novel coronavirus, nCoV-2019”. CEPI-US. Consulted on January 26, 2020.
- www.pharmalicensing.com “Molecular Clamp: a Novel Protein Vaccine for Influenza, RSV, Ebola and Other Human and Veterinary Viruses”. ↑. Accessed January 26, 2020.
- ↑ Insider, James Hennessy, Business. “Australia’s Been Asked to Make a Coronavirus Vaccine at ‘Unprecedented Speed’. ScienceAlert-gb. Accessed 26 January 2020.
- ↑ “Inovio, Moderna score CEPI funding for vaccine work against deadly coronavirus”. FiercePharma. Accessed January 26, 2020.
- ↑ “Infectious Diseases | Moderna, Inc.”. www.modernatx.com. Accessed January 26, 2020.
- ↑ a b “Local Biotech Company Developing Coronavirus Vaccine.” NBC 7 San Diego-US. Accessed January 26, 2020.
- ↑https://jpost.com/HEALTH-SCIENCE/Israeli-scientists-In-three-weeks-we-will-have-coronavirus-vaccine-619101 Israeli scientists: ‘In a few weeks, we will have coronavirus vaccine
- ↑ Karen Carey (February 26, 2020). “Increasing number of biopharma drugs target COVID-19 as virus spreads”. BioWorld. Accessed 1 March 2020.
- ↑ Jaimy Lee (7 March 2020). “These nine companies are working on coronavirus treatments or vaccines – here’s where things stand”. MarketWatch. Accessed 7 March 2020.
- ↑ “Gilead assessing Ebola drug as possible coronavirus treatment”. Reuters. 23 January 2020. Reuters. 23 January 2020.
- ↑ Zhao, Yuning (30 January 2020). “Three drugs fairly effective on novel coronavirus at cellular level”. China News Service. Archived since original 29 January 2020. Accessed 1 February 2020.
- ↑. Wang, Manli; Cao, Ruiyuan; Zhang, Leike; Yang, Xinglou; Liu, Jia; Xu, Mingyue; Shi, Zhengli; Hu, Zhihong et al. “Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research 30: 269-71. ISSN 1748-7838. PMC 7054408. PMID 32020029. doi:10.1038/s41422-020-0282-0. Consulted 27 February 2020.
- ↑ “Cocktail of flu, HIV drugs appears to help fight coronavirus: Thai doctors”. Reuters. February 3, 2020. Accessed 5 February 2020.
- ↑ “Coronavirus: Thailand has apparent treatment success with antiviral drug cocktail”. South China Morning Post. 2 February 2020. Archived since original February 2, 2020. Accessed 3 February 2020.
- ↑ “China Applies for Patent for Potential Coronavirus Drug”. Time. Archived since original February 6, 2020. Accessed 5 February 2020.
- ↑ “China lab seeks patent on use of Gilead’s coronavirus treatment”. Physician’s Weekly. 5 February 2020. Filed since original 5 February 2020. Accessed 5 February 2020.
- ↑ “我国学者在抗2019新型冠状病毒药物筛选方面取得重要进展”. whiov.ac.cn(in Chinese). Archived since original 5 February 2020. Accessed 5 February 2020.
- ↑ “China remdesivir test, as a coronavirus drug”. aristeguinoticias.com. Accessed February 8, 2020.
- ↑. “China lab seeks patent on use of Gilead’s coronavirus treatment”. Physician’s Weekly. February 5, 2020. Accessed 5 February 2020.
- ↑ “China Applies for Patent for Potential Coronavirus Drug”. Time. Accessed 5 February 2020.
- ↑ Li G, De Clercq E (March 2020). “Therapeutic options for the 2019 novel coronavirus (2019-nCoV)”. Nature Reviews. Drug Discovery 19 (3): 149-150. PMID 32127666. doi:10.1038/d41573-020-00016-0.
- ↑ Beeching, Nicholas J.; Fletcher, Tom E.; Fowler, Robert (2020). “BMJ Best Practices: COVID-19. BMJ.
- ↑ “AIFA and Gilead announce that Italy is among the countries that will be testing the antiviral drug for the treatment of COVID-19”. aifa.gov.it(en it-IT). Accessed on 19 March 2020.
- ↑”Russia’s Ministry of Health names three drugs that can treat new Chinese coronavirus”. Accessed 10 February 2020.
- ↑”China tests Russian anti-viral drug which might treat coronavirus as Moscow warns of possible ‘mass outbreak'”. RT news. Accessed 11 February 2020.
- ↑”China Tests Russian Antiviral Drug Which Might Treat Coronavirus As Moscow Warns Of Possible ‘Mass Outbreak'”. Zambia Reports. Accessed 11 February 2020.
- ↑ Cao, Bin; Wang, Yeming; Wen, Danning; Liu, Wen; Wang, Jingli; Fan, Guohui; Ruan, Lianguo; Song, Bin et al. “A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. New England Journal of Medicine 0 (0): null. ISSN 0028-4793. doi:10.1056/NEJMoa2001282. Consulted on March 22, 2020.
- ↑ “Researchers find two new drugs that can effectively inhibit coronavirus”. news.cgtn.com Accessed 19 February 2020.
- ↑ “As China Clamps Down on Negative News, Quarantines on Land and Sea”. The New York Times. 5 February 2020. ISSN 0362-4331. Accessed 19 February 2020.
- ↑ Gao, Jianjun; Tian, Zhenxue; Yang, Xu (2020). “Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends. advpub. doi:10.5582/bst.2020.01047. Retrieved February 27, 2020.
- ↑ Sebastian, Nieves (27 February 2020). “Covid-19: Chloroquine phosphate could treat pneumonia”. Gaceta Médica. Consulted on February 29, 2020.
- ↑ Wenzhong, Liu; Hualan, Li (10 March 2020). COVID-19 Disease: ORF8 and Surface Glycoprotein Inhibit Heme Metabolism by Binding to Porphyrin. doi:10.26434/chemrxiv.11938173.v2.
- ↑ Andersen PI; Ianevski A; Lysvand H; Vitkauskiene A; Oksenych V; Bjoras M; Telling K; Lutsar I; Dumpis U; Irie Y; Tenson T. “Discovery and Development of Safe-in-Man Broad-Spectrum Antiviral Agents”. Preprints 0 (0): null. doi:10.20944/preprints201910.0144.v4.
- ↑ Reina, J; Reina, N (April 2017). “Favipiravir, a new concept of antiviral drug against influenza viruses”. Spanish Journal of Chemotherapy 30 (2): 79-83. PMID 28176519. Consulted on March 18, 2020.
- ↑ “Antiviral drug Favipiravir shows clinical efficacy against COVID-19 pneumonia, according to official”. spanish.xinhuanet.com. Xinhua. March 17, 2020. Accessed March 18, 2020.
- ↑ “Coronavirus treatment: China announces results of favipiravir antiviral”. Medical Writing. March 17, 2020. Accessed March 18, 2020.
- ↑ Hoffmann, Markus; Kleine-Weber, Hannah; Krüger, Nadine; Müller, Marcel; Drosten, Christian; Pöhlmann, Stefan (31 January 2020). “The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus ACE2 receptor and the cellular protease TMPRSS2 for entry into target cells”. bioRxiv (preprint). doi:10.1101/2020.01.31.929042.
- ↑ Iwata-Yoshikawa, Naoko; Okamura, Tadashi; Shimizu, Yukiko; Hasegawa, Hideki; Takeda, Makoto; Nagata, Noriyo (March 15, 2019) “TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection”. Journal of Virology 93 (6). PMC 6401451. PMID 30626688. doi:10.1128/JVI.01815-18.
- ↑ Yao, Xueting; Ye, Fei; Zhang, Miao; Cui, Cheng; Huang, Baoying; Niu, Peihua; Liu, Xu; Zhao, Li et al. “In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)”. Clinical Infectious Diseases. doi:10.1093/cid/ciaa237. Accessed March 18, 2020.
- ↑ Liu, Jia; Cao, Ruiyuan; Xu, Mingyue; Wang, Xi; Zhang, Huanyu; Hu, Hengrui; Li, Yufeng; Hu, Zhihong et al. “Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro”. Cell Discovery 6 (1): 1-4. ISSN 2056-5968. doi:10.1038/s41421-020-0156-0. Accessed March 22, 2020.
- ↑ France, Connexion. “French researcher posts successful Covid-19 drug trial“. www.connexionfrance.com Accessed 18 March 2020.
- ↑”Coronavirus : diagnoses and traits ! Premiers résultats pour la chloroquine – IHU” (in fr-FR). Accessed on March 18, 2020.
- ↑ France, Connexion. “French researcher posts successful Covid-19 drug trial“. www.connexionfrance.com (en anglais). Accessed 18 March 2020.
- ↑ France, Connexion. “French researcher posts successful Covid-19 drug trial”. www.connexionfrance.com (in English). Accessed 18 March 2020.
- www.heraldsun.com.au “‘Cure’ found for coronavirus in Australia”. ↑ 16 March 2020. Accessed 18 March 2020.
- www.heraldsun.com.au “‘Cure’ found for coronavirus in Australia”. ↑ 16 March 2020. Accessed 18 March 2020.
- ↑ “Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an openlabel non-randomized clinical trial”.
- ↑”Hydroxychloroquine and azithromycin as a treatment of COVID-19 – IHU” (en fr-FR). Accessed 18 March 2020.
- ↑ Effective Treatment of Severe COVID-19 Patients with Tocilizumab. ChinaXiv.org. 5 March 2020. doi:10.12074/202003.00026. Accessed 14 March 2020.
- ↑ Liu, Roxanne; Miller, Josh (3 March 2020). “China approves use of Roche drug in battle against coronavirus complications. Reuters. Reuters. Accessed 14 March 2020.
- ↑ “Coronavirus, via libera dell’Aifa al farmaco antiartrite efficace su 3 pazienti e a un antivirale: test in 5 centri”. Il Messaggero(in Italian). Accessed on March 14, 2020.
- ↑ “3 patients get better on arthritis drug”. March 5, 2020. Accessed 14 March 2020.
- ↑ a b c Riou, Julien; Hauser, Anthony; Counotte, Michel J.; Althaus, Christian L. (March 6, 2020). “Adjusted age-specific case fatality ratio during the COVID-19 epidemic in Hubei, China, January and February 2020. medRxiv: 2020.03.04.20031104. doi:10.1101/2020.03.04.20031104. Accessed 17 March 2020.
- ↑ Ruan Q; Yang K; Wang W; Jiang L; Song J (March 2020) “Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China”. Intensive Care Medicine. PMID 32125452. doi:10.1007/s00134-020-05991-x.
- ↑ “How doctors can potentially significantly reduce the number of deaths from Covid-19”. Vox. March 12, 2020. Accessed March 14, 2020.
- ↑”China turns Roche arthritis drug Actemra against COVID-19 in new treatment guidelines”. FiercePharma. Accessed 14 March 2020.
- ↑”Northwell Health Initiates Clinical Trials of 2 COVID-19 Drugs”. 21 March 2020.
- ↑ a b c d Casadevall A; Pirofski LA (March 2020). “The convalescent sera option for containing COVID-19.” The Journal of Clinical Investigation. PMID 32167489. doi:10.1172/JCI138003.
- ↑ Pearce, Katie (13 March 2020). “Antibodies from COVID-19 survivors could be used to treat patients, protect those at risk: Infusions of antibody-laden blood have been used with reported success in prior outbreaks, including the SARS epidemic and the 1918 flu pandemic”. The Hub at Johns Hopkins University. Accessed March 14, 2020.
- ↑ “Coronavirus: Vir Biotechnology and Novavax announce vaccine plans-GB”. Accessed 26 January 2020.
- ↑ Wang, Chunyan; Li, Wentao; Drabek, Dubravka; Okba, Nisreen M. A.; Haperen, Rien van; Osterhaus, Albert D. M. E.; Kuppeveld, Frank J. M. van; Haagmans, Bart L. et al. (12 March 2020). “A human monoclonal 1 antibody blocking SARS-CoV-2 infection”. bioRxiv: 2020.03.11.987958. doi:10.1101/2020.03.11.987958. Accessed 15 March 2020.
- ↑ “Unieke vondst in Erasmus MC: antilichaam tegen corona”. Erasmus Magazine(in nl-NL). 13 March 2020. Accessed 15 March 2020.
- ↑ “WHO Director-General’s statement on the advice of the IHR Emergency Committee on Novel Coronavirus”. who.int.
- ↑ Wang, Weier; Tang, Jianming; Wei, Fangqiang (2020). “Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China”. Journal of Medical Virology. PMID 31994742. doi:10.1002/jmv.25689.
- ↑ a b“Vital Surveillances: The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) – China, 2020”.
- ↑ “New-type coronavirus causes pneumonia in Wuhan: expert – Xinhua | English.news.cn”. www.xinhuanet.com. Accessed 9 January 2020.
- ↑ World Health Organization. “Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)”. pp. 11-12. Accessed 5 March 2020.
- ↑ “Live from WHO HQ – Daily Press Briefing on COVID-19 –Coronavirus 03MARCH2020”. World Health Organization Youtube channel. March 3, 2020. Accessed March 16, 2020.
- ↑ Fottrell, Quentin. “Coronavirus fatality rates vary dramatically depending on age, gender, medical history and country. MarketWatch. Accessed 5 March 2020.
- ↑ “Epidemiological Characteristics of 2143 Pediatric Patients With 2019 Coronavirus Disease in China”.
- ↑ “Coronavirus testing: how are the hardest-hit countries responding?”. Financial Times. 11 March 2020. Accessed March 13, 2020.
- ↑ World Health Organization (WHO) (ed.). “Coronavirus disease outbreak (COVID-19): guidance for the public”. Archived since original 16 March 2020. Accessed 16 March 2020.
- ↑”COVID-19 Prevention & Treatment”. United States: Centers for Disease Control and Prevention. February 11, 2020. Accessed February 25, 2020.
- ↑”Advice for public”. World Health Organisation. Archived since original 26 January 2020. Accessed 10 February 2020.
- ↑ “What to do if you are sick with 2019 Novel Coronavirus (2019-nCoV)”. United States: Centers for Disease Control and Prevention. February 11, 2020. Accessed February 13, 2020.
- ↑ Tubio, Silvia (March 11, 2020). “It is a crime to spread the coronavirus intentionally or by not taking preventive measures”. ABC Sevilla (Abc.es). Consulted on March 13, 2020.
- ↑”COVID-19 Thematic Website, Together, We Fight the Virus, COVID-19″. Hong Kong government coronavirus website. Consulted on February 22, 2020.
- ↑”COVID-19 Information for Travel”. United States: Centers for Disease Control and Prevention. February 11, 2020. Accessed February 25, 2020.
- ↑ a b“Severe Respiratory Disease associated with a Novel Infectious Agent.” Hong Kong: Centre for Health Protection, Department of Health. Accessed 1 February 2020.
- ↑ a b c“Coronavirus disease (COVID-19) advice for the public: Myth busters.” World Health Organization (WHO). Accessed 26 February 2020.
- ↑ “China bans trade, consumption of wild animals due to coronavirus”. CNBC. 25 February 2020. Accessed 2 March 2020.
- ↑ “Coronavirus Disease 2019 (COVID-19)”. United States: Centers for Disease Control and Prevention. February 11, 2020. Accessed February 25, 2020.
- ↑ CDC (February 11, 2020). “Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. Accessed March 2, 2020.
- ↑ a b“Advice for public.” World Health Organization (WHO). Accessed 8 February 2020.
- ↑ a b“Coronavirus Disease Outbreak (COVID-19): Guidelines for the Public.” World Health Organization (WHO). Accessed 15 March 2020.
- ↑ Home. “Novel Coronavirus”. Health Protection Surveillance Centre. Accessed on 27 February 2020.
- ↑”Updates on Wuhan Coronavirus (2019-nCoV) Local Situation”. Singapore: Ministry of Health. Accessed 1 February 2020.
- ↑ a b c d“Advice on the use of masks the community, during home care and in health care settings in the context of the novel coronavirus (2019-nCoV) outbreak”. World Health Organization. Accessed 21 February 2020.
- ↑”2019-nCoV: What the Public Should Do”. United States: Centers for Disease Control and Prevention. February 4, 2020. Accessed February 5, 2020.
- “↑”Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings. United States: Centers for Disease Control and Prevention. February 6, 2020. Accessed February 8, 2020.
- “↑. “As Hongkongers clamour for surgical masks, 25,000 stolen from warehouse. Hong Kong: South China Morning Post. 31 January 2020. Accessed 1 February 2020.
- ↑ Takahashi, Ryusei. “Amid virus outbreak, Japan stores scramble to meet demand for face masks”. Japan Times. Accessed 1 February 2020.
- ↑ munsan (31 January 2020). “Wuhan virus: Who needs to wear a mask and what’s the proper way to wear it?”. Singapore: The Straits Times. Accessed 1 February 2020.
- ↑ Chia, Rachel Genevieve. “These 12 Twitter posts show the insane queues for masks in Singapore, Shanghai and Hong Kong, which are all sold out.” Business Insider Singapore. Accessed 1 February 2020.
- ↑ Harun, Hana Naz; Teh, Athira Yusof; Solhi, Farah (31 January 2020). “Demand for face masks, hand sanitisers soars”. Malaysia: New Straits Times. Accessed 1 February 2020.
- ↑ CDC (11 February 2020). “What to Do If You Are Sick With Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. Accessed February 26, 2020.
- ↑ “Common questions about coronavirus (COVID-19)”. nhs.uk 24 February 2020. Accessed 26 February 2020.
- ↑https://www.gov.uk/guidance/wuhan-novel-coronavirus-information-for-the-public
- ↑”New coronavirus (2019-nCoV): guidelines for the public”.
- ↑ “Coronavirus”. www.who.int Accessed 16 January 2020.
- ↑ Lyons, Amanda (January 17, 2020). “Novel coronavirus: What GPs need to know.” RACGP.
- ↑ “Advice for public”. www.who.int(in English). Accessed 8 March 2020.
- ↑ “Singapore: The Model for COVID-19 Response?”. www.medpagetoday.com(in English). 5 March 2020. Accessed 8 March 2020.
- ↑ Ivana Kottasová; Lindsay Isaac. “Italy shuts all schools over coronavirus outbreak. CNN. Accessed 8 March 2020.
- ↑ “Coronavirus (COVID-19): What is social distancing? – Public health matters”. publichealthmatters.blog.gov.uk. Accessed 9 March 2020.
- ↑ Pueyo, Tomas (12 March 2020). “Coronavirus: Why You Must Act Now”. Medium. Accessed 12 March 2020.
- ↑ CDC (11 February 2020). “People at Risk for Serious Illness from COVID-19”. Centers for Disease Control and Prevention. Accessed 8 March 2020.
- ↑ Fetters, Ashley (10 March 2020). “When Keeping Your Distance Is the Best Way to Show You Care. The Atlantic. Accessed 12 March 2020.
- ↑”Watch out! Spitting in public places too can spread infections” (en-IN). February 14, 2020. Accessed March 12, 2020.
- ↑ “Coronavirus: The psychological effects of quarantining a city”. The British Medical Journal. 24 January 2020. Accessed 10 February 2020.
- ↑ Xiang, Yu-Tao; Yang, Yuan; Li, Wen; Zhang, Ling; Zhang, Qinge; Cheung, Teris; Ng, Chee H (4 February 2020). “Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. The Lancet Psychiatry: S2215036620300468. doi:10.1016/S2215-0366(20)30046-8.
- ↑ Kang, Lijun; Li, Yi; Hu, Shaohua; Chen, Min; Yang, Can; Yang, Bing Xiang; Wang, Ying; Hu, Jianbo; Lai, Jianbo; Ma, Xiancang; Chen, Jun (5 February 2020) “The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus”. The Lancet Psychiatry: S221503662030047X. doi:10.1016/S2215-0366(20)30047-X.
External links
- Coronavirus disease outbreak (COVID-19) on the World Health Organization website
- New coronavirus disease, COVID-19 on the website of the Spanish Ministry of Health